Posted: Feburary 2023
New Publication:
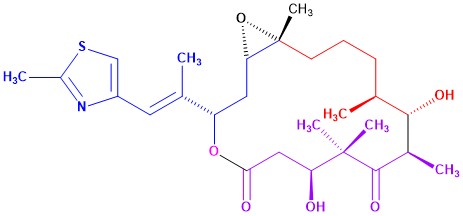
Process for the Manufacture of Ixabepilone
United States Patent
Jalal Haddad & Sourena Nadji
Patent No.: US 10,464,942 B2
Date of Patent: Nov. 5, 2019
We Have Moved!
We have Moved!
WE HAVE MOVED, WE JUSDT EXPANDED OUR PRESENCE IN SAINT LOUIS!
Upcoming Publication
Spaced-heparinoids Provisional Patent soon to be submitted!
Heparan Sulfate in Literature
Interesting Articles
Perlecan: a review of its role in neurologic and musculoskeletal disease
DOI: 10.3389/fphys.2023.1189731
TREM2 inhibition triggers antitumor cell activity of myeloid cells in glioblastoma
Benjamin T. Himes, ….and Ian F. Parne
https://pubmed.ncbi.nlm.nih.gov/37172094/
Initial Data from Allarity’s Phase 2 Trial of IXEMPRA® Indicate Potential for Improved Clinical Benefit in DRP®-Selected Metastatic Breast Cancer Patients
07/05/2023
Boston , MA (July 5, 2023) — Allarity Therapeutics, Inc. (“Allarity” or the “Company”) (Nasdaq: ALLR), a clinical-stage pharmaceutical company developing novel oncology therapeutics together with drug-specific DRP® companion diagnostics for personalized cancer care, today announced initial results from its European Phase 2 clinical trial evaluating the efficacy of IXEMPRA® in metastatic breast cancer (mBC) patients selected with the DRP®-IXEMPRA® companion diagnostic (CDx) candidate. Researchers prescreened mBC patients using Allarity’s DRP®-IXEMPRA® CDx, a complex transcriptomic signature comprising multiple mRNA biomarkers of drug response/resistance. Patients were assigned a DRP®-score, and those with scores above 67% were selected for treatment with IXEMPRA®.
Of the 36 patients screened with the DRP®-IXEMPRA® CDx, investigators identified five DRP® positive patients. Among the evaluable patients assessed up to the data evaluation cut-off, there were promising signs of clinical benefit in four out of four evaluable cases:
- One partial responder (PR) (tumor shrinkage of 66%).
- One partial responder (PR) (tumor shrinkage of 59%).
- One patient experienced 24 weeks of stable disease.
- One patient experienced 19 weeks of stable disease.
“ We are enthusiastic about these promising very early trial results since the observed clinical benefit rate exceed s what has been historically observed for IXEMPRA ® treatment without the DRP ® – IXEMPRA ® CDx patient selection. While still early, these data suggest that the use of the DRP ® -IXEMPRA ® CDx for patient selection and treatment may help identify mBC patients most able to benefit from this course of treatment . Accordingly , the DRP ® -IXEMPRA ® CDx , if approved, may provid e clinicians with an important diagnostic to guide patient treatment in this hard-to-treat population, “ said Marie Foegh, M.D., Chief Medical Officer of Allarity.
The study is in a very early stage of an ongoing open-label, single-arm trial, at multiple sites in Europe, evaluating the anti-tumor effect of IXEMPRA® in patients with locally recurrent or metastatic breast cancer after previous chemotherapies, including a taxane and an anthracycline. The included patients received a maximum of three prior lines of chemotherapies in the metastatic setting. Allarity recently amended the clinical trial protocol, lowering the DRP® cut-off score in order to include more likely responder patients while still excluding those unlikely to respond to the drug. The ultimate objective is to further refine the DRP®-IXEMPRA® CDx criteria and broaden the enrollment of mBC patients who may substantially benefit from this treatment. The Company anticipates an additional interim data readout before the end of this year.
The DRP®-IXEMPRA® CDx is a transcriptomic signature comprising 191 mRNA biomarkers that are collectively predictive of tumor sensitivity or resistance to IXEMPRA®. Using the DRP® CDx to select likely responder patients while excluding likely resistant ones, Allarity aims to improve the benefit-risk ratio of IXEMPRA® in metastatic or locally advanced breast cancer. The U.S. FDA-approved IXEMPRA® label currently indicates a monotherapy efficacy with an objective response rate (ORR) of 12.4% and a clinical benefit rate (CBR) of 24.8% in metastatic or locally advanced breast cancer. However, the initial data from the ongoing DRP®-guided Phase 2 study of IXEMPRA® suggest that the DRP®-IXEMPRA® CDx may identify a subset of patients who potentially have an improved ORR and CBR as compared to monotherapy efficacy indicated by the U.S. FDA-approved label for the drug. The DRP®-IXEMPRA® CDx is a clinical stage companion diagnostic candidate and has not yet been approved by the U.S. FDA or the EMEA. Early trial results are insufficient to show statistical significance and may not be a reliable indicator of subsequent trial results based on a larger patient population.
IXEMPRA® was originally developed by Bristol Myers Squibb and is approved for metastatic breast cancer patients in the U.S., where it is marketed by R-PHARM U.S., LLC. Allarity has the exclusive option rights for the development and commercialization of IXEMPRA® in Europe.
Allarity’s Chief Executive Officer, James G. Cullem, further stated, “We are encouraged by these promising, early clinical data suggesting that mBC patients selected for treatment with IXEMPRA® using our DRP® companion diagnostic candidate for the drug mayhave substantially improved clinical benefit versus unselected patients. Allarity looks forward to fully enrolling and completing our ongoing Phase 2 trial for IXEMPRA®, and remains enthusiastic about advancing this program towards market approval in Europe and, if approved, providing European mBC patients with first–time access to this beneficial drug.”